
The 7 step approach to Fever in returned travellers:
- Consider serious infection
- Consider the need for isolation
- Elicit important points in travel and medical history
- Do a careful physical examination
- Order the correct investigations
- Start treatment early
- Notify the disease according to CHP instructions
Introduction
Travel is getting easier and more affordable for more and more people. Many destinations previously considered as ‘exotic’ are now reachable thanks to the low cost of air travel and to the internet information explosion. Nowadays, even the most remote regions of the world can be reached within 36 hours, which is less than the incubation period of most infectious diseases. The implication is that a person can get infected whilst travelling, and come back to his/her home country or to another country before manifesting signs and symptoms of a particular infectious disease which is not present at the place where medical attention is sought. It follows that in an international city like Hong Kong, we may encounter virtually any kinds of infectious disease.
Knowledge on the likely etiology of fever
10-42% and 15-70% respectively of travelers to any destination and to tropical settings experience ill health, either while abroad or on returning home [1]. One particular group of ‘travelers’ are those who have migrated to another country and then return to their original country to visit friends & relatives (VFR). These VFR may not consider themselves ‘travelers’ but they have lost a lot of the immunity they previously had to certain diseases present in their original country, for example, more than 70% of malaria cases in the US and UK occurred in VFR [2,3].
Infectious diseases that are common throughout the world e.g. streptococcal throat infection, influenza, urinary tract infection, appendicitis, diverticulitis and others may also occur in a returned traveler. Hence the differential diagnosis for fever in a returned traveler should include these “ubiquitous” conditions as well as ‘exotic’ infections.
Fever is a common presenting symptom in returned travelers and accounted for 28% of cases in one series [4]. Common causes of fever in returned travelers include malaria, dengue, enteric fever and rickettsial infections. Malaria is uncommon in Hong Kong. In the period January 2017 to September 2019, the Centre for Health Protection (CHP) of the Department of Health recorded a total of 65 cases of malaria. All cases, except one unclassified case, were imported from endemic countries (Nigeria 15 cases, India 11 cases and Uganda 5 cases) [5].
1. Consider serious infection
When the patient first presents to the outpatient clinic or emergency room, the first point of contact (POC) physician should perform a rapid assessment. The general status, vital signs and mental status of the patient should be assessed. When 2 or more of 3 criteria in the quick sequential organ function assessment (qSOFA) are met, this indicates a severe infection requiring urgent attention. These 3 criteria include (a) systolic blood pressure of equal or less than 100mmHg; (b) high respiratory rate of equal or more than 22 breaths/min and (c) Glasgow Coma Scale of less than 15 [6]. Rapid empirical treatment and intensive care support should be considered if such criteria are met [7]. In-patient care and prompt referral to an infectious disease specialist is recommended. It is noted that even if the criteria of qSOFA are not met and the patient has been to a malarial endemic area then urgent investigation to rule in or rule out malaria should be done as falciparum malaria may progress from relatively well to fatal in a matter of hours. The maxim for fever in the returning traveler is “IT IS MALARIA UNTIL PROVEN IT IS NOT MALARIA”. We also recommend that a lower threshold for admission to hospital is needed for immunocompromised travelers who are prone to rapid disease progression. Additionally, immunocompromised travelers often have a reduced protection from inactivated vaccines and most will not have been given live vaccines.
2. Consider the need for isolation
After the SARS outbreak and now with the COVID-19 pandemic, there is a generalized increased awareness of the importance of infection control in healthcare settings. There is triage system for major emerging diseases in place in the emergency departments of hospitals in Hong Kong [8]. Similar triage systems were not often in place in private clinics in Hong Kong until the COVID-19 pandemic. This triage system helps screen out high risk patients presenting with severe respiratory infection who have so called TOCC (travel, occupational, contact and clustering) criteria for COVID-19, Middle East Respiratory Syndrome (MERS) and Avian influenza for isolation and rapid diagnosis. Additionally, the presence of rash, diarrhea or hemorrhage should prompt the clinician to consider the need to initiate appropriate infection control precautions. Thus, for example, the exposure history to animals or sick people should be elicited in febrile patients who have travelled to endemic areas for viral hemorrhagic fevers (VHF). There may be very serious public health implications if a case of VHF is missed. A detailed travel history is essential. The only epidemiological link may be that of visiting a place with a current outbreak. The Travel Health Service website of the Department of Health is a good resource for information about recent outbreaks [9] as is the Geosentinel webpage hosted by the International Society of Travel Medicine which is constantly being updated [10].
3. Elicit important points in travel and medical history
(a) First ask about the travel history
The travel history should be taken in all patients, whether febrile or not. Many doctors fail to take a good travel history and consequently miss major infectious diseases such as malaria. Even if the travel history has been elicited a life-threatening infection can be missed if the physician does not think of the diagnosis and test for it. This is especially true if the presenting symptoms are atypical, for example, malaria presenting with diarrhea and vomiting and mis-diagnosed as gastroenteritis. The patient had even told the physician that she had traveled to sub-Saharan Africa! Patients may also present outside the usual incubation period e.g. a case of vivax malaria presented several months after returning from Ethiopia. It is therefore important to elicit the travel history over the last year and longer. Case 4 below illustrates an infectious disease of long incubation period! Three questions should always be considered (1) WHERE DID YOU GO? (2) WHEN DID YOU GO? (3) WHAT DISEASES ARE POSSIBLE?
(b) When was the travel?
The time of travel may suggest the possibility of a particular infectious disease e.g. melioidosis occurs mainly in the rainy season because the organisms are located in the mud. Leptospirosis occurs in warmer months in temperate climates and in the rainy season in the tropics.
The time from possible exposure to the onset of symptoms helps to narrow down the diagnosis. The incubation period of infectious diseases associated with travel can be classified as short (<=10 days), intermediate (11-28 days) and long (>28 days). Table 1 shows a list of infectious diseases together with their incubation periods.
(c) Exposure during travel
Because patients may not volunteer information it is important that the doctor asks the following specific questions:
| 1.Place of stay: Ask whether the patient visited urban or rural areas. A backpacker is exposed to different diseases compared to a tourist staying in a 5-star hotel e.g. Chagas disease and Hantavirus disease would be unlikely diagnoses in non-rural travelers. 2. Food and drink: Ask whether the patient has drunk any unclean, tap and surface water or unpasteurized milk; or eaten any raw or undercooked meat, fish or vegetables. 3. Sexual history: Ask about the nature of sexual encounters, the number of partners and whether barrier precautions have been used and used properly. It is not uncommon for travelers to lower their inhibitions while they are on a trip and engage in sexual activities with new sexual partners. 4. Activity history: Ask whether there has been any exposure to soil and water, including recreational activities like hiking, rafting, swimming and hunting. Ask if there has been exposure to insects and animals including birds and any history of animal bites or licks. 5. Blood exposure: Ask whether the patient received any health care when traveling including injections, acupuncture, dental work, surgery or blood transfusion. Ask about tattoos or body piercing. 6. Exposure to sick people: Ask whether the patient has taken care of patients or refugees. |
Table 2 showed major infectious diseases associated with different exposures and the list is not exhaustive.
Table 1: Infectious diseases and their common incubation periods
Short incubation period (<=10days) | Intermediate incubation period (11-28 days) | Long incubation period (>28 days) |
Bacterial | ||
• Anthrax | • Bartonellosis (can be long) | • Bartonellosis |
Viral | ||
• Arboviral disease: | • Rabies (can be very long) • Viral haemorrhagic fevers (usually short) | • CMV (can be intermediate) |
Parasitic | ||
• Malaria (never <7 days) | • Amoebic liver abscess (can be long) | • Amoebic liver abscess (can be intermediate) |
Fungal | ||
Histoplasmosis | Histoplasmosis | |
Table 2: Infections associated with specific exposures
| EXPOSURE | INFECTIOUS DISEASES |
|---|---|
| Taking unclean food and water | Traveler’s diarrhea, Shigella, Salmonella, Enteric fever (typhoid and paratyphoid fever), Campylobacter, Helminth infections, Giardia, Cryptosporidium; Hepatitis A and E; Amoebic dysentery or amoebic liver abscess |
| Eating undercooked meat | Campylobacter, Salmonella, E coli O157, Toxoplasma, Trichinella |
| Eating undercooked shellfish | Hepatitis A & E, Vibrio, Clonorchis |
| Drinking unpasteurized milk | Brucella, Listeria, Salmonella, Tuberculosis |
| Sexual contacts | HIV, Hepatitis B and C, syphilis, gonorrhea, chlamydia, HSV, HPV and others |
| Fresh water contact | Leptospirosis, Schistosomiasis |
| Mosquito bites | Malaria, Dengue, Japanese encephalitis, West Nile virus, Zika, Chikungunya, Yellow fever etc |
| Tick or Mite bites or walking through tall grass or woods | Rickettsia, Babesia, Lyme disease, Tick-borne encephalitis |
| Animal and bird contacts | Q fever, Brucella, Anthrax, Tularemia, MERS, Avian flu |
| Caving | Rabies, Histoplasmosis |
| Air conditioning systems, showers | Legionella |
| Healthcare | Ebola, Tuberculosis, Antibiotic resistant bacteria. |
| Blood exposure | Hepatitis B, C, HIV, Viral hemorrhagic fever (VHF), Malaria |
(d) Use of malaria prophylaxis and vaccines: it is important to obtain a detailed vaccination history and whether or not the patient took malaria prophylaxis. I f the patient did take malaria prophylaxis, ask whether or not they were fully compliant with the prophylaxis and whether they took the correct prophylaxis bearing in mind that the correct prophylaxis depends upon the resistance spectrum in the area(s) visited. Malaria or vaccine preventable diseases should not be excluded based on the prophylaxis or vaccination history.
4. Careful physical examination of the patient
Detailed physical examination of the patient cannot be overemphasized. Sometimes pathognomonic signs may be hidden in sites not usually examined. The authors have seen eschar of Rickettsial infection hidden under the underwear in the groin regions, on the scrotum or penis or under the axilla. A rash can be localized instead of generalized and may be missed on casual physical examination. Important physical signs and associated infections are listed in Table 3.
Table 3: Key physical signs suggestive of cause of fever
| Clinical sign | Possible infections (not exhaustive) |
| Jaundice | Viral hepatitis, malaria, leptospirosis, yellow fever |
| Conjunctival haemorrhage | Leptospirosis, dengue |
| Meningism | Japanese encephalitis or other arthropod-borne encephalitis. Aseptic meningitis in acute HIV infection, Leptospirosis, Lyme disease, Rabies |
| Maculopapular rash | Dengue, Enteric fever (typhoid and paratyphoid fever), Rickettsia, acute HIV, Syphilis, Gonococcal infection, Chikungunya |
| Petechial rash | Rickettsia, Meningococcal infection, Leptospirosis, Viral hemorrhagic fever (VHF), Dengue |
| Urticarial rash | Acute schistosomiasis (Katayama fever), Strongyloides |
| Eschar | Rickettsia, Scrub typhus, Anthrax |
| Ulcers | Leishmaniasis (cutaneous or mucocutaneous), Mycobacteria, Anthrax |
| Erythema chronicum migrans (ECM) | Lyme disease |
| Localized lymphadenopathy | African trypanosomiasis, Chagas disease, Cat scratch fever, Herpes simplex (primary), Plague, Rickettsia, Syphilis, Lymphogranuloma venereum (LGV) |
| Generalized lymphadenopathy | Brucellosis, HIV (acute), Visceral leishmaniasis (Kala-azar), Leptospirosis, Relapsing fever (Borreliosis), Rickettsia, Trypanosomiasis |
| Splenomegaly | Malaria, Enteric fever, Visceral leishmaniasis |
| Hepatomegaly | Leptospirosis, Enteric fever, Viral hepatitis |
5. Order the correct investigations
Initial investigations will be the same as for a sepsis workup for any febrile illness, with the addition of testing for malaria, and include complete blood count, blood cultures, liver and renal function tests, serum inflammatory markers (C-reactive protein, ESR), procalcitonin, urine & stool microscopy and culture and a chest X-ray. Blood cultures, preferably several sets, should be taken before giving antibiotics. The white cell count is the most useful. Malaria testing should include both a blood smear and rapid antigen test. Rapid antigen testing is of high sensitivity especially for falciparum malaria. Malaria testing may need to be done several times before a diagnosis is made especially if the patient has taken malaria prophylaxis and there is only a low-level parasitemia. Table 4 shows the typical findings of the laboratory tests in selected infectious diseases. If there is reason to suspect specific infectious disease based on history, physical examination or initial investigation, one should order additional investigations shown in Table 5. At the initial presentation serum should be saved as several infectious diseases need two serum specimens (at presentation and during convalescence) of paired serum to confirm diagnosis. Saved serum is also useful in cases where the diagnosis was only considered retrospectively.
Table 4: Common abnormalities on initial ‘routine’ sepsis workup investigations:
| Laboratory abnormalities | Infectious diseases |
|---|---|
| NEVER FORGET: Malaria smear and rapid antigen test | ALWAYS rule out malaria! This may need testing several times especially if malaria prophylaxis has been taken. |
| Complete blood count: | |
| WBC raised | Sepsis, Leptospirosis, Campylobacter, Tularaemia, Legionella, Tick borne relapsing fever, Louse borne relapsing fever, Amoebic liver abscess |
| WBC low or normal | Sepsis, Enteric fever, Brucellosis, Rickettsia, Ehrlichiosis, Toxoplasmosis, Q fever, Malaria, Kalaazar, many viruses |
| Atypical lymphocytosis | Many viruses |
| Eosinophilia | Helminthic (Fasciola, Filaria, Trichinella, Schistosoma, Strongyloides etc) |
| Anemia | Malaria, Babesia, Ehrlichiosis |
| Thrombocytopenia | Malaria, Dengue, Viral hemorrhagic fever (VHF) |
| Elevated liver transaminases | Enteric fever, Hepatitis, Malaria, Dengue, CMV, EBV |
Table 5: Key diagnostic tests for selected travel-related infectious diseases
| Bacterial | Key diagnostic test(s) |
| Anthrax | Culture, Gram stain, PCR |
| Bacteremia caused by cosmopolitan infections | Blood culture |
| Bacterial gastroenteritis | Culture, Stool PCR |
| Bartonellosis | Culture, serology, PCR |
| Brucellosis | Culture (blood or bone marrow), serology |
| Ehrlichiosis | Serology, buffy coat examination, PCR |
| Enteric fever (typhoid and paratyphoid fever) | Blood culture (Widal test is of limited value) |
| Legionellosis | PCR, urine antigen testing, culture |
| Leptospirosis | Serology, PCR, culture |
| Lyme disease | Serology |
| Melioidosis | Culture, PCR |
| Meningococcal infection | Blood & CSF culture, CSF analysis and PCR |
| Plague | Culture, serology |
| Psittacosis | Serology, PCR |
| Q fever | Serology, PCR, culture (Level 3 lab) |
| Relapsing fever | Direct visualization on blood film, PCR |
| Syphilis | Serology |
| Typhus/Rickettsial infections | Serology, PCR |
| Tuberculosis | Smear, culture, molecular methods |
| Tularaemia | Serology, PCR, culture (with caution) |
| Yersiniosis | Culture, Stool PCR |
| Viral | |
| Chikungunya fever | PCR, serology |
| CMV | PCR, serology, culture |
| Dengue | PCR, serology |
| Haemorrhagic fever with renal syndrome (HFRS) caused by hantavirus | Serology |
| Hepatitis B | Serology |
| Hepatitis C | Serology; HCV RNA |
| HIV | Serology; p24 antigen; HIV RNA |
| Influenza including avian influenza | PCR; antigen detection assay less preferred |
| Japanese encephalitis | Serology |
| Rabies | PCR (saliva); serology (blood and CSF); skin biopsy |
| Yellow Fever | PCR, serology |
| Zika | PCR, serology |
| Parasitic | |
| Amebic liver abscess | Serology, Imaging, Aspirate stain, PCR, Antigen testing |
| Fascioliasis | Microscopy of stool, serology |
| Filariasis | Blood smear, Antigen and antibody tests |
| Malaria | Blood smear, rapid diagnostic tests |
| Schistosomiasis | Microscopy of urine and stool or biopsy, serology |
| Toxocariasis | Serology, imaging |
| Toxoplasmosis | Serology |
| Trichinosis | Serology, muscle biopsy |
| Trypanosomiasis | Microscopy, serology |
| Fungal | |
| Histoplasmosis | Culture, microscopy, serology |
6. Start treatment early
Treatment depends upon the diagnosis and specific treatment also depends upon a knowledge of the resistance spectrum in the country of acquisition. Table 6 lists conditions and scenarios in which empiric antibiotic may be warranted. In some cases it is important to start treatment based upon a presumptive diagnosis before there is laboratory confirmation. This is especially true for Rickettsial infections where laboratory confirmation may take a day or two and where early treatment with doxycycline may save lives.
Table 6: Pathogen directed/empirical therapy for common travel-related infectious diseases
Bacterial | Empiric Treatment |
Fever and severe bloody diarrhea within 3 weeks of exposure: suspect bacterial gastroenteritis | Azithromycin |
Undifferentiated fever within 3 weeks after arrival in a high incidence enteric fever area: suspect enteric fever (typhoid and paratyphoid fever) | Ceftriaxone |
Fever and headache ± rash or eschar within 2 weeks of tick exposure: suspect Rickettsial or Scrub typhus infection | Doxycycline |
Flu like symptoms ± jaundice ± hepatorenal failure within 2 weeks of fresh water exposure: suspect acute leptospirosis | Ceftriaxone or doxycycline |
Viral | |
Nil | |
Parasitic | |
Fever, urticarial rash and eosinophilia with 4-8 weeks after fresh water exposure: suspect acute schistosomiasis | Symptomatic and eventually Praziquantel ± Steroid |
Fever, abdominal pain and liver ultrasound showing abscess OR bloody diarrhea weeks -months or even years after travel to endemic areas: suspect amoebic liver abscess (ALA) or amoebic dysentery | Metronidazole |
If there is no cause found for the fever or if the patient is quite ill then the patient should be referred to a specialist in infectious disease or tropical medicine. All cases of Falciparum malaria should be immediately referred as the condition can rapidly deteriorate to death in a matter of hours. Other forms of malaria should also be referred. Vivax malaria, for example, although going by the synonym of “Benign tertian malaria” can often be fatal. In addition all patients with suspected infected scoring 2 or above on qSOFA as described at paragraph 1 need urgent referral.
7. Notify the Centre for Health Protection of notifiable diseases and diseases of topical public health concern
For the latest list of infectious diseases which are statutorily notifiable in Hong Kong, please refer to updated information on CHP Central Notification Office (CENO) online [11].
There is also a list of communicable diseases of topical public health concern (DPC). Though not specified in the First Schedule to the Prevention and Control of Disease Ordinance, medical practitioners are urged to report suspected or confirmed cases of the following diseases to CENO for arrangement of investigation and control as appropriate. Table 7 showed all currently notifiable diseases and disease of topical public health concern.
Conclusion
A proper history and physical examination i s of paramount importance in making the specific diagnosis in a returning traveler with fever. Even if the diagnosis is not obvious by careful attention to the “7 steps” described, an important diagnosis will not be missed. A knowledge of the incubation period, exposure risks and clinical and laboratory presentations gives clues to the diagnosis. Targeted investigations at the right time confirm the diagnosis. Finally, there is no substitute for experience and specialist referral may make a difference in patient outcome.
Table 7: List of notifiable diseases and diseases of publicconcern (DPC) in Hong Kong
Notifiable diseases | Diseases of public concern (DPC) |
Bacterial | |
Anthrax | Brucellosis |
Viral | |
Acute poliomyelitis | Acute flaccid |
Parasitic | |
Amoebic dysentery | Cryptosporidiosis |
Others | |
Creutzfeldt-Jakob disease | Myiasis |
Cases studies
CASE 1
M/37 management consultant Indian. Working in Hong Kong, family in Hyderabad.
He spent 3 weeks in the city of Hyderabad in India before coming back to Hong Kong. He did not visit the countryside during his trip. He gave a one-week history of fever and chills two days after returning to Hong Kong. He attended AED of a public hospital and did not wait due to a long queue. At a private clinic he was told that he had influenza. Because he was feeling very weak, he attended another clinic and was told he had hepatitis. Eventually he was admitted to a private hospital where he was diagnosed with falciparum malaria. He was found to have hyperparasitemia with a parasite count of 20%. He had diarrhea, hypoglycemia and was deeply jaundiced. Bilirubin was 326μmol/l and increased to 606μmol/l on day 6 of admission. There was only mild elevation of ALT and clotting profile and ammonia levels were normal. Renal function was mildly impaired but the creatinine rose to 308μmol/l on Day 6 and he needed continuous venousvenous haemodialysis. His WBC increased from 6.5 × 109/L on admission to 20 × 109/L on Day 3. He was covered with broad spectrum antibiotics for suspected secondary Gram-negative sepsis. His respiratory function deteriorated and he developed Acute Respiratory Distress Syndrome (ARDS) and was intubated and later needed extracorporeal membrane oxygenation (ECMO). He was given artensunate intravenously and his parasite count fell to 3% by day 3 and to zero on day 6. He had deafness on recovery but responded well to cochlear implants.
Diagnosis: Malaria
Learning lessons:
Malaria can masquerade as many common infectious diseases, even gastroenteritis or hepatitis! Hence all travelers who have visited malaria-endemic areas (even ‘urban’ areas) and who develop symptoms whilst on the trip or after return should have a blood smear and a rapid antigen test to rule out malaria. Clinical deterioration and death can occur within hours in falciparum malaria. Some patients who have visited a country to visit friends and relatives (VFR) may not consider their trip a ‘travel’ and such travel should be explicitly elicited in the history.
CASE 2
M/50 British pilot living in Hong Kong
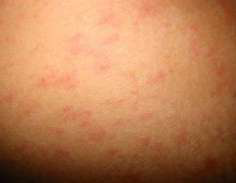
He spent a holiday in Thailand at a resort island at the end of Nov 2019. One week after returning to Hong Kong he complained of fever, generalized malaise and myalgia. A general practitioner diagnosed an influenza-like illness and he was given symptomatic treatment. Soon after fever onset he noted a non- itchy rash over his back and trunk. He attended another doctor 3 days later for persistent fever and rash. Dengue PCR and antigen test confirmed a diagnosis of dengue. Pictures 1 & 2 show his rash.
Diagnosis: Dengue
Learning lessons:
Dengue is the most common mosquito borne viral infection worldwide. Its clinical presentation can range from asymptomatic to a mild febrile illness to severe hemorrhagic fever with or without shock. It often presents with the sudden onset of fever ≥38.5°C. The fever lasts 3 to 7 days. It may be accompanied by headache, eye pain, myalgia, and arthralgia. A transient macular or maculopapular rash occurs around 2-5 days after fever onset in around 50% of cases and is seen over the face, thorax, abdomen and extremities. Hemorrhagic manifestations include petechiae and ecchymoses. Other clinically significant bleeding such as haematemesis, heavy menstrual bleeding and epistaxis may occur. Physical examination may show lymphadenopathy, conjunctival injection, petechiae and bruising. Pictures 3 & 4 show a middle-aged Chinese male admitted to hospital for hypotension and fever. The pictures were taken after the use of a tourniquet for blood taking. It is important to watch out for signs of systemic vascular leakage and this usually occurs around days 3-7 of the illness around the time of defervescence and the appearance of the rash. Signs and symptoms include shock, bleeding, vomiting, lethargy, severe abdominal pain, development of pleural effusion and/or ascites.
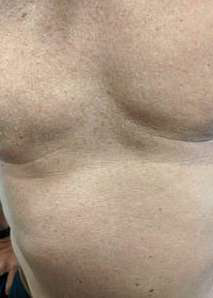
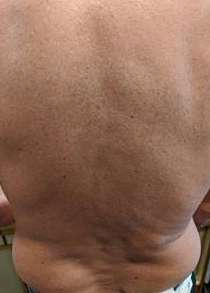
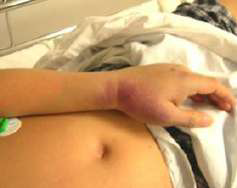
CASE 3
M/24. Local postgraduate student
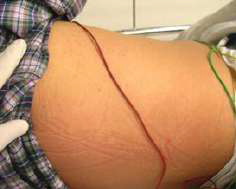
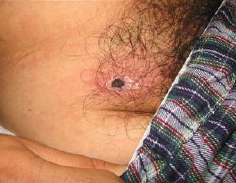
This patient presented with a one-week history of malaise and a five-day history of high fever. One week before symptom onset he had trekked in Chiang Mai, Thailand. On examination there was a discrete maculopapular rash over the back and trunk (see picture 5). There was non-tender cervical, axillary and inguinal lymphadenopathy. There was a blackish lesion under his underwear near his groin (see picture 6). His WBC was 4.9 × 109/L Neutrophil 56.5%, lymphocyte 24%, monocyte 12%, atypical lymphocytes 4%, Hb 13.5 g/dL, platelet 130 × 109/L. Rickettsial serology was negative on admission. He was treated with doxycycline 200mg followed by 100mg bd. His fever decreased within 1 day of starting treatment and continued to decrease in linear manner. Rickettsial paired serology done on presentation and after 10 days showed an increase of titer of 1:1024 against Orientia tsutsugamushi.
Diagnosis: Scrub typhus
Learning lessons:
- Look hard for the pathognomonic eschar which is often found in skin folds.
- If the diagnosis is suspected, start the patient on doxycycline before Rickettsial serology is back as Rickettsial infection can be fatal and the early use of doxycycline can be life-saving.
- Serial serology needs time to become positive and a single negative serology on admission does not rule out infection.
CASE 4
M/20. Student from USA
This patient gave a three-day history of severe frontal headache, fever with chills and rigors. He had vomited five times on the day prior to admission. He also complained of neck stiffness, back pain and myalgia. He had been given ceftriaxone 2g intravenously once several hours before transfer to our hospital because a diagnosis of bacterial meningitis was considered. Direct questioning revealed no sore throat, ear discomfort, coryza, cough, chest pain, breathlessness or wheezing. Vision and hearing were normal. He denied diarrhea, constipation or abdominal discomfort. Micturition was normal. He had no arthralgia or skin rash. Two weeks before admission he had been in Hawaii and two weeks ago before that was in San Diego, his home base. He had had good past health.
On examination: Temperature 41 degree Celsius. There was muscle tenderness over his thighs and calves. His conjunctivae were suffused bilaterally. There was no lymphadenopathy, rash or eschar. He was fully orientated. Kernig’s sign was negative and his neck was only mildly stiff at the limit of flexion. Neurological examination was otherwise unremarkable. He had sinus tachycardia of 130/min. Examination of cardiovascular, respiratory systems and abdomen were normal. CT brain was normal. Chest X Ray was normal. WBC was 7.3 ×109/L (neutrophil 96%) and platelets were 117×109/L. AST was 76 U/L (normal <37). Creatinine was 117 μmol/L. CPK was 1900 U/L (35-232). Widal test was negative except paratyphoid B 1/180. Weil Felix was negative. Dengue and malaria tests were negative. Lumbar puncture was a traumatic tap and showed an increase in red and white cells and protein. CSF glucose was normal. CSG Gram stain and CSF latex tests for bacterial meningitis were negative. Intravenous ceftriaxone was continued and the fever and headache resolved. Leptospira serology on admission was negative and seroconverted to positive 10
days after admission.
Diagnosis: Leptospirosis
Learning lessons:
The elevation CPK in the absence of previous intramuscular injection, conjunctival suffusion, myalgia, mild increase in liver transaminases and creatinine all suggest a diagnosis of leptospirosis. The patient probably acquired the infection from water or mud while he was playing sports in Hawaii. Leptospirosis may occur in cities in flood situations and in recent years there have been large outbreaks in Mumbai and Jakarta. It is also common in those who have crawled through mud, e.g soldiers on exercise or athletes undergoing “Iron Man” or “Action Asia” events. One of us had nine patients admitted to a private hospital at the same time. All these patients were from Hong Kong and had participated in an “Iron Man” event in Subic Bay in the Philippines. Important differential diagnoses include meningococcal meningitis and severe dengue.
CASE 5
M/30. Tour guide from Hong Kong
The patient was a tour guide who did outbound tours to Asian countries including Japan and South-east Asia. He presented with fever, rash and headache and was admitted through the Accident & Emergency Department as a case of suspected measles. On examination there was a generalized erythematous maculopapular rash over his truck and limbs. There was bilateral cervical lymphadenopathy and moderate neck rigidity. Kernig sign was positive. The white blood count was 11.5 × 109/L with lymphocytic predominance of 68% and 10% atypical lymphocytes. Hemoglobin and platelets were normal. Liver and renal function tests were normal. Blood and urine cultures were negative. Chest X ray was normal. There was splenomegaly on ultrasound. An MRI of the brain showed meningeal enhancement. Cerebrospinal fluid (CSF) showed protein 0.92 g/L and 40 cells/mm3 with 100% mononuclear cells. Serological tests for hepatitis A, B, and C viruses, cytomegalovirus, and syphilis were negative. Toxoplasmosis and Epstein-Barr virus IgG antibodies were positive. ELISA (enzyme linked immune sorbent assay) for HIV was positive. Western blot (WB) showed a positive band for HIV p24. HIV viral load was 2,680,000 copies/mL and the CD4 T cell count was 433 cells/uL. The findings were compatible with aseptic meningitis in primary HIV seroconversion illness. The patient was heterosexual and admitted to having unprotected sex with a commercial sex worker in Tokyo.
Diagnosis: Acute HIV seroconversion illness
Learning lessons:
Acute HIV infection may mimic many infectious diseases including infectious mononucleosis, severe Streptococcal pharyngitis, secondary syphilis and toxoplasmosis [12]. The diagnosis is missed in as many as 75% of patients because of a low index of suspicion [13]. This is especially so in patients over 50 years old [14].
Patients who return from the tropics with fever are frequently assumed to have tropical infections such as malaria, typhoid etc. One study found that acute HIV infection was the cause in 3% of such febrile illness [15]. It is important that healthcare workers adopt a non-judgmental attitude when asking the sexual history [16]. Early diagnosis of acute HIV infection followed by use of antiretroviral therapy
CASE 6
M/45. IT engineer
The patient was an Indian who had lived in Hong Kong for 10 years. He went back to India Christmas 2019 to visit friends & relatives (VFR). He presented in mid-January 2020 with one week of left lower abdominal pain. The pain had become more constant in the 2 days before presentation and he had developed fever and chills. WBC on admission was 5.4 x 109/L (neutrophil 71%, lymphocyte 19%, monocyte 7%). CT abdomen on admission showed many mesenteric lymph nodes in the right iliac fossa and upper central abdomen. Blood culture on admission grew Salmonella paratyphi sensitive to ceftriaxone and resistant to fluoroquinolones. Widal test was negative. Malaria smear was negative. He was given two weeks of ceftriaxone and his fever gradually resolved after the first 5 days of treatment.
Diagnosis: Paratyphoid fever
Learning lessons:
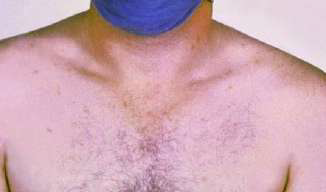
In patient with febrile illness, especially with a normal or lowish WBC and a pulse temperature deficit, enteric fever should be on the differential diagnosis. It can mimic, and may even present as, an acute abdomen. Lymphadenitis is due to the bacteria entering the blood stream triggering the systemic reaction in the body. Blood cultures taken before giving antibiotics is the diagnostic gold standard. The Widal test is unreliable and is effectively obsolete. Rose spots are pink blanching papules 2-8 mm in size found in 33% of patients with enteric fever (typhoid or paratyphoid) (Picture 7). Characteristically, rose spots are seen in untreated typhoid fever between the second and fourth week of illness and last 3-5 days. They are due to bacterial emboli to the skin spreading through the bloodstream. Salmonella can be cultured from these spots. Salmonella typhi or Salmonella paratyphi in South Asia and South-east Asia is showing an increasingly high resistance to fluoroquinolones and, if the diagnosis is suspected, ceftriaxone should be the first-line antibiotic.
CASE 7
F/33. Local reporter from Hong Kong
About six weeks before presentation the patient went on an ‘adventure travel’ trip with a friend lasting two weeks to East Africa. Prior to the trip she attended a local travel clinic and had her vaccinations updated and was given anti-malarial prophylaxis. She was well whilst in Africa. She gave a two-day history of fever, cough and urticaria starting about three weeks after her return to Hong Kong. A malaria smear was negative. The eosinophil count was increased to 1.35 ×109/L. Schistosomal serology was weakly positive. On questioning, she admitted to swimming in Lake Malawi on two occasions. Her friend had also swum in Lake Malawi and her friend had complained of pruritus after swimming (“swimmer’s itch”) but dismissed this as allergy (it was in fact due to Cercarial dermatitis- due to Schistosoma larvae entering the skin). The patient did not have a rash. It was decided not to give her steroids before treatment. She was treated with praziquantel (PZQ) in two divided doses over one day with a total dose of 40mg/kg. Praziquantel was given only after her acute symptoms had resolved since PZQ is not effective against the larval stages of schistosomiasis. It has no activity in the first 21 days after infection, low activity at day 28 and almost 100% activity at day 52. On the other hand prolonged delay in giving PZQ – e.g up to 12 weeks after infection, may increase the risk of developing neuroschistosomiasis. Her acute symptoms were due to Katayama fever – a hypersensitivity reaction to migrating schistosomules. Her eosinophil count normalized with treatment and repeat serology was strongly positive. She was given one further dose of PZQ 3 months after the first dose to ensure eradication. Follow up serology after one year was still strongly positive but serology can remain positive for years after schistosome eradication.
Diagnosis: acute schistosomiasis (Katayama fever)
Learning lessons:
Think of acute schistosomiasis (Katayama fever) in someone presenting with:
- At least one of the following: fever, cough, rash, diarrhea.
- A recent history of fresh water exposure in an area where schistosomiasis is endemic.
- Positive schistosomal serology, either at presentation or on follow-up.
- An increased eosinophil count at some point during the illness.
- Symptoms not attributable to any other condition.
Exposure in Lake Malawi occurred in over 50% of confirmed patients in one series [17] and is said to be a ‘tell-tale’ point in history! One of us holds the maxim: “You cannot swim in Lake Malawi without getting Schistosomiasis”.
CASE 8
M/56. British working in Hong Kong
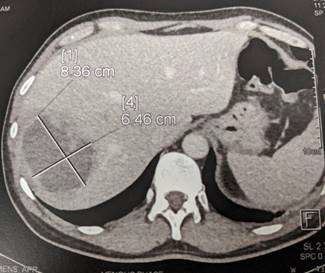
The patient was referred with a one-week history of night fever, malaise and decreased appetite. He had attended outpatients at a private hospital and was empirically given a course of levofloxacin. His illness continued unchanged and he reattended outpatients and was found to have a neutrophil count of 20.8 × 109/L and a raised CRP of 320 mg/dL. He was referred for further management. He had no abdominal pain and bowel motions were normal. Liver function tests showed bilirubin 6 μmol/L (<20), alkaline phosphatase 130 U/L (<120), alanine transaminase 25 U/L (<35). A CT abdomen showed the presence of an 8cm abscess in the right lobe of his liver and an inflamed appendix without perforation (picture 8). He underwent laparoscopic appendectomy and drainage of the abscess. The amoebic titer was positive. Entamoeba histolytica PCR was positive in both the aspirate from the liver abscess and in his stool. His liver abscess was cured with a course of metronidazole. This was followed by paramomycin to eradicate the luminal amoeba in the colon.
Diagnosis: Amoebic liver abscess
Learning lessons:
It is easy not to consider infectious diseases with prolonged incubation periods in the differential diagnosis of fevers. Most of us, especially in this time of COVID-19, focus mainly on the recent travel history in the last 2-4 weeks. Always remember to take extended travel histories [18].
Amoebic liver abscess can present as pyrexia of unknown origin (PUO) without any localizing signs. Think of this differential diagnosis in anyone presenting with fever, malaise and neutrophilia with mild liver function derangement.
CASE 9
F/22. Filipino domestic helper
A domestic helper with good past health arrived in Hong Kong 6 weeks prior to presentation at the Accident & Emergency Department of a public hospital. She complained of pain on the right calf for 2 days at the site of a healed wound. She was discharged with analgesics. She reattended the next day with fever, chills, myalgia, nausea, vomiting and epigastric pain. A history of having been bitten by a stray dog one week prior to coming to Hong Kong was elicited. She was admitted and two days after admission developed right lower limb numbness and weakness and loss of knee jerks. The next day she became irritable, mildly confused and suffered hydrophobia. There was sinus tachycardia of 140/min. CT Brain showed no obvious lesion. Lumbar puncture was done and CSF opening pressure was 24cm H2O. CSF protein and glucose were normal. RBC 26 per cc and WBC 185 per cc (4% polymorph and 96% lymphocyte). Gram stain and bacterial antigen, cryptococcal antigen, AFB smear were all negative. She was transferred to ICU and succumbed soon afterwards.
Diagnosis: Rabies
Learning lessons:
Rabies is a condition that once seen never forgotten! It is rare in Hong Kong but common in the Philippines. The history of dog bite in her rural home was elicited only at her second attendance on direct questioning and was not volunteered by the patient. Patients often do not know the significance of the history and direct questioning is important. The last imported case in Hong Kong was 2015. It is thus very rarely seen in Hong Kong and a high level of suspicion is needed.
References
- Fink D et al. Fever in the returning traveler. BMJ 2018; 360:j5773 doi:10.1136/bmj.5773
- UK Government. Malaria in the UK. Annual report, 2016. Accessed on 15 Mar 2020 at https://www.gov.uk/government/publications/malaria-in-theuk- annual-report
- Cullen KA et al. Centers for Disease Control and Prevention (CDC). Malaria Surveillance – United States, 2013. MMWR Surveill Summ 2016;65:1-22.
- Wilson ME et al. Fever in returned travelers: result from the GeoSentinel Surveillance Network. Clin Infect Dis 2007; 44: 1560.
- Centre for Health Protection, HK. Review of mixed malarial infection in Hong Kong. Communicable Disease Watch. 2019; 16: 107-108. Accessed on 15 Mar 2020 at https://www.chp.gov.hk/files/pdf/cdw_v16_22.pdf
- Seymour CW et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and shock (Sepsis -3): JAMA 2016;315:762-74.
- Howell MD, Davis AM. Management of sepsis and septic shock. JAMA 2017;317:847-8.
- Wong ATY et al. From SARS to Avian Influenza Preparedness in Hong Kong. Clinical Infectious Diseases 2017; 64 (suppl 2): S98–S104.
- Travel Health Service, Department of Health. https://www.travelhealth.gov. hk/eindex.html
- International Society of Travel Medicine, Geosentinel website. https://www. istm.org/geosentinel
- Centre for Health Protection, DH. CENO online assessed at https://cdis.chp. gov.hk/CDIS_CENO_ONLINE/ceno.html
- Wong TY, So MK, Primary HIV infection: heightened awareness needed. HKMJ 2001; 7: 205-8.
- Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339:33–9.
- AIDS among persons aged > or = 50 years—United States, 1991–1996. MMWR Morb Mortal Wkly Rep. 1998;47:21–7.
- Doherty JF, Grant AD, Byceson AD. Fever as the presenting complaint of travellers returning from the tropics. QJM. 1995;88:277–81.
- US CDC. A guide to taking a sexual history. https://www.cdc.gov/std/ treatment/sexualhistory.pdf
- Logan S, et al. Acute Schistosomiasis in Travelers: 14 Years’ Experience at the Hospital for Tropical Diseases, London. Am J Trop Med Hyg. 2013 Jun 5; 88(6): 1032–1034.
- HKSAR Government. Queen Queen Mary Hospital announces severe case of malaria. https://www.info.gov.hk/gia/general/201704/03/ P2017040300879.htm
- Bell DJ. Fever in the returning traveller. J R Coll Physicians Edinb 2012; 42, 43-6.doi: 10.4997
- Johnston V, et al. Fever in returned travellers presenting in the United Kingdom: Recommendations for investigation and initial management. Journal of Infection 2009; 59:1-18.
- Thwaites GE, et al. Approach to Fever in Returning Traveler. NEJM 2017; 376:548-60.
Forward from《Hong Kong Medical Association CME Bulletin》
