Andrew T. Y. Wong,1 Hong Chen,1 Shao-haei Liu,2 Enoch K. Hsu,1 Kristine S. Luk,3 Christopher K. C. Lai,4 Regina F. Y. Chan,5 Owen T. Y. Tsang,6 K. W. Choi,7 Y. W. Kwan,8 Anna Y. H. Tong,9 Vincent C. C. Cheng,10 and Dominic N. C. Tsang11; on behalf of the Central Committee on Infectious Diseases and Emergency Response, Hospital Authority, Hong Kong Special Administrative Region
1Infectious Disease Control Training Centre, Hospital Authority Head Office; 2Infection, Emergency & Contingency, Quality and Safety Division, Hospital Authority Head Office; 3Department of Pathology, Princess Margaret Hospital; 4Department of Pathology, Queen Elizabeth Hospital; 5Infection Control Team, United Christian Hospital; 6Hospital Authority Infectious Disease Centre, Princess Margaret Hospital; 7Department of Medicine, Alice Ho Miu Ling Nethersole Hospital; 8Department of Paediatrics and Adolescent Medicine, Princess Margaret Hospital; 9Information Technology and Health Informatics Division, Hospital Authority Head Office; 10Department of Microbiology, Queen Mary Hospital; 11Chief Infection Control Officer Office, Hospital Authority Head Office, Hong Kong Special Administrative Region, China
The first human H5N1 case was diagnosed in Hong Kong in 1997. Since then, experience in effective preparedness strategies that target novel influenza viruses has expanded. Here, we report on avian influenza preparedness in public hospitals in Hong Kong to illustrate policies and practices associated with control of emerging infectious diseases. The Hong Kong government’s risk-based preparedness plan for influenza pandemics includes 3 response levels for command, control, and coordination frameworks for territory- wide responses. The tiered levels of alert, serious, and emergency response enable early detection based on epidemiological exposure followed by initiation of a care bundle. Information technology, laboratory preparedness, clinical and public health management, and infection control preparedness provide a comprehensive and generalizable preparedness plan for emerging infectious diseases.
Keywords. avian influenza; emerging infectious diseases; preparedness.
After emergence of the first case of severe acute respiratory syndrome (SARS) in Hong Kong in 2003, major system changes on how to handle emerging infectious diseases (EIDs) have been formulated [1]. In the same year, a SARS Expert Review recommended establishment of the Center for Health Protection (CHP) under the Department of Health to increase the capacity to deal with future resurgences of SARS and other EIDs [2]. The functions of the CHP include comprehensive public health surveillance related to communicable diseases, contingency planning for disease outbreaks, and building capacity and professional expertise. As part of establishment of the CHP, professional staff, including doctors and nurses, who work in public hospitals also work in branches responsible for infection prevention and control, epidemiological investigation, and outbreak control. This model facilitates cross-fertilization of expertise between hospitals and the public health environment.
Here, we present the journey of a patient with avian influenza (AI) to illustrate the measures and actions taken, as guided by the Hospital Authority’s (HA’s) preparedness plan for influenza pandemic. We also describe our surge capacity when the number of AI cases increases to a predefined critical level. With the constant worldwide pandemic threat, planning and preparedness before the next pandemic are critical for an effective response. The information is of value to other countries in formulation of their preparedness plans.
A Patient With Avian Influenza
Patient A was a 68-year-old man who presented to an accident and emergency department (AED) of a regional hospital with fever and cough in December 2013. Present illness was significant for poultry exposure 5 days earlier in Shenzhen, China. After triaging at the AED, he was admitted to the hospital’s airborne infection isolation room (AIIR). His nasopharyngeal specimen tested positive by polymerase chain reaction (PCR) for influenza A (H9N2) virus. He was transferred to the Infectious Disease Center (IDC) of Princess Margaret Hospital, and the CHP was notified of the potential detection of AI. The response level was raised from alert to serious according to the HA preparedness plan for influenza pandemic on the same day. The CHP and the hospital infection control team (ICT) initiated epidemiological investigations of contacts in the community and hospital. Enhanced disease surveillance, diagnostic laboratory support, port health measures including health surveillance on travellers at immigration control points, and health education relating to AI were activated.
Overall Command Structure of the Preparedness Plan
Continuous preparedness plans are important to minimize the disastrous effects of transmissible microbial agents in healthcare settings. During the SARS outbreak, there were reports
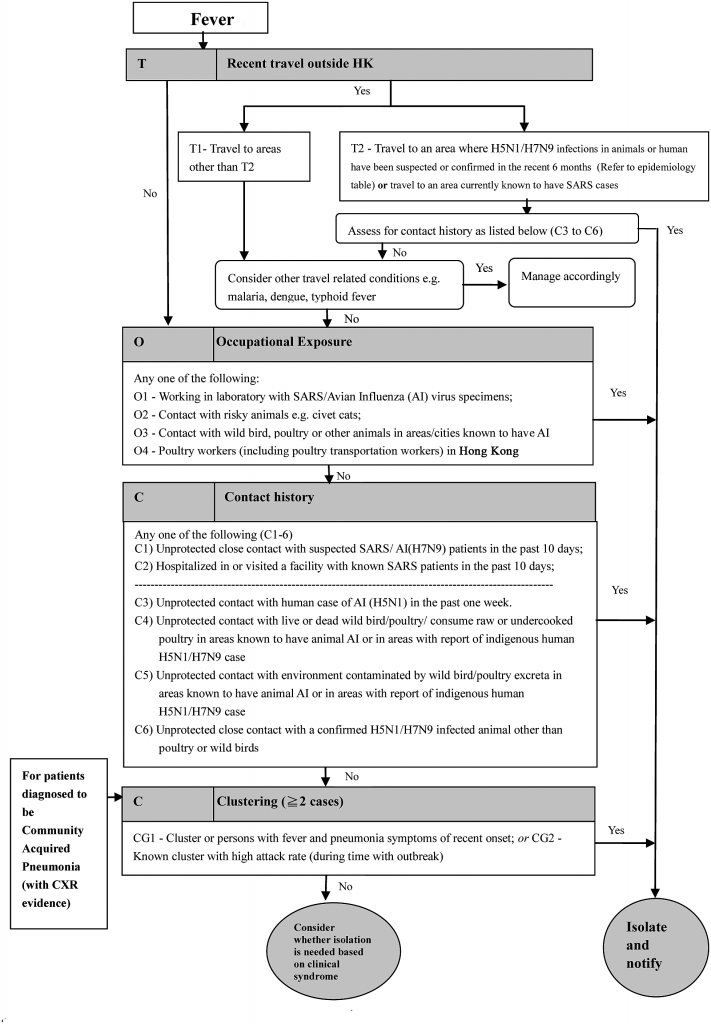
Figure 1. FTOCC (Fever >38°C, Travel to areas with confirmed human avian influenza infection, Occupational exposure, Contact history with confirmed cases or poultry/ wild birds, and Clustering of multiple patients) triage assessment for febrile patients with respiratory symptoms in an accident and emergency department and general outpatient clinic in Hospital Authority hospitals. Abbreviations: AED, accident and emergency department; CHP, Center for Health Protection; HA, hospital authority; HR, human resources; ICU, intensive care unit; ILI, influenza-like illness; LTCF, long term care facility; OPD, outpatient department; PHLSB, public health laboratory services branch; PPE, personal protection equipment; RT-PCR, reverse-transcription polymerase chain reaction. Source: Infection, Emergency & Contingency, Quality and Safety Division, HA Head Office. Internal Information Page on Avian Influenza A (H7N9).
of unclear roles and responsibilities among the health authorities [2]. Stemming from the subsequently devised SARS response system, the Hong Kong Special Administrative Region (HKSAR) government preparedness plan for influenza pandemic includes the following 3 response levels: alert, serious, and emergency, corresponding to the evolving epidemiology
From SARS to Avian Influenza Preparedness in Hong Kong • CID 2017:64 (Suppl 2) • S99
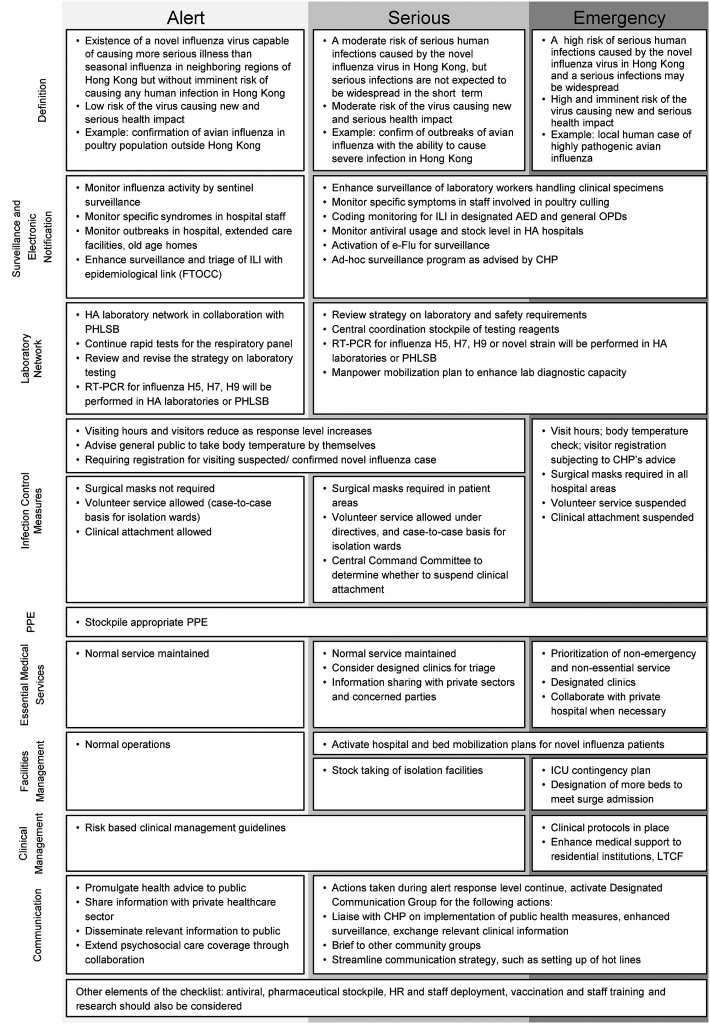
Figure 2. Checklist for preparedness for avian influenza in hospitals. Abbreviations: AI, avian influenza; CXR, chest X-ray; SARS, severe acute respiratory syndrome. Source: Working Group for Reviewing Contingency Plans for AI & SARS. HA Preparedness Plan for Influenza Pandemic. 2012. Chief Infection Control Officer Office. HA Infection Control Plan (Influenza Pandemic). 2014.
that was first formulated in January 2005 and modified and enriched in the following years [3]. These response levels are based on risk assessment of novel influenza that may affect Hong Kong and its health impact on the community (Figure 1). The different response levels provide the command, control, and coordination framework for territory-wide response. There are corresponding actions to be taken by public hospitals during different response levels. The HA’s response to AI generally follows the HKSAR government preparedness plans, so this greatly facilitates communication with the public. To implement and disseminate the disease control policies, there is a Central Committee on Infectious Disease and Emergency Response. Its membership includes representatives from related divisions from the HA head office, infection control officers (ICOs) of 7 hospital clusters, and representatives from related clinical specialties and the CHP [4]. In addition to preparedness plans for influenza pandemic, the 3 response levels are also adopted in HKSAR preparedness and response plans for other EIDs including Middle East respiratory syndrome (MERS), Ebola virus disease (EVD), and Zika virus infection.
Early Case Detection, Surveillance, and Notification
Detection of at-risk exposure relies on “epidemiological linkage” upon history taking. After the SARS epidemic in 2003, the acronym FTOCC (Fever >38°C, Travel to areas with confirmed human AI infection, Occupational exposure, Contact history with confirmed cases or poultry/wild birds, and Clustering of multiple patients) was developed as part of a triage flow chart used when screening patients with epidemiological exposure to EIDs [5] (Figure 1). The list of areas affected by AI is regularly updated and has been made available online since 2005 (http:// www.chp.gov.hk/en/guideline1_year/441/332.html). Over the years, SARS, AI, MERS, and EVD have been added to the flow chart to act as “gate-keepers” at the AED. Patients who fulfill clinical and epidemiological criteria for a suspected case are cared for using a bundle of recommended measures. For AI, the bundle includes placement in an AIIR for contact, droplet, and airborne precautions; rapid molecular testing of respiratory specimens; and notification to the CHP.
Enhanced laboratory surveillance enables early detection of silent carriers of potential AI cases in the community. Respiratory specimens from patients with community acquired pneumonia who have epidemiological risk factors are automatically tested for AI using PCR. A recent study showed that 33% of patients with H7N9 infection did not have poultry or wet market (market that sells fresh meat, poultry, and fish) contact [6]. In our review, for the 23 patients with AI from March 2007 to April 2016 (2 H5N1, 16 H7N9, 5 H9N2), 15 patients had visited a wet market, and 20 patients had travelled to affected areas. The enhanced laboratory surveillance with PCR helps to identify cases that may otherwise be missed by the epidemiological triage that relies on history taking alone.
Information technology (IT) plays an important role in EID management. The HA leverages its integrated clinical IT infrastructure to build a comprehensive clinical management system (CMS) that supports all frontline clinical activities in public hospitals and clinics [7]. Timely notification to the CHP can be done with the electronic notifiable diseases and outbreak reporting system, which is integrated into the CMS. A contact tracing system of the patient administration system has been developed to aid in the automatic generation of a list of patients who potentially have contacted the index case within the specified time period and who need proper outbreak control management such as antiviral prophylaxis. The SARS experience showed that transmission among healthcare workers in healthcare facilities was not uncommon [8]. In 2005, the HA developed a web-based “staff early sickness alert system” based on staff absenteeism and sickness information to facilitate early detection of an unusual rise or trend. In the past 10 years, a number of novel AI cases (2 H5N1, 16 H7N9, and 5 H9N2) and variant H3N2 have been successfully detected, isolated, and treated with this strategy.
Infection Control Preparedness
There has been an infection control infrastructure in public hospitals that comprise ICOs and infection control nurses (ICNs) in each hospital since the HA was established in 1991. The system became more comprehensive after the SARS epidemic in 2003, with a link to the ICN system to enhance communication with each clinical unit. The staffing at ICN was enhanced after SARS. The current full-time equivalent ICN-to-bed ratio in acute care hospitals is about 1:250.
In 2014, the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommended a combination of contact, droplet, and airborne precautions in view of the high morbidity and mortality of novel influenza infections [9, 10]. We segregate suspected AI cases in AIIRs accordingly. Isolation facilities in all acute care hospitals were upgraded after SARS. Currently there are 1400 airborne infection isolation beds in HA hospitals. The technical design and ventilation parameters are in accordance with CDC recommendations.
There are clear guidelines on the use of personal protection equipment (PPE) by staff for patient care under different response levels. Each hospital has to keep a 3-month stockpile of PPE for the possibility of peak use capacity [4]. Fit-testing and training on usage of N95 respirators are offered to all healthcare workers. Use of an N95 respirator is recommended for all aerosol-generating procedures.
Healthcare workers are required to have refresher infection- control training every 24 months and to have their updated training records kept by the hospital management for independent review. Forums and just-on-time sessions are provided by the hospital ICT in addition to e-learning programs. In the workplace, there are poster reminders on PPE donning and doffing sequences. The ICT regularly performs hand hygiene and environmental hygiene audits, with feedback to the clinical units. Regular infectious disease drills and exercises at the actual scene with participation of different stakeholders have been organized to test the response of hospitals and healthcare workers.
The Australian Council on Healthcare Standards was introduced into Hong Kong for hospital accreditation in 2010. Public Downloaded from https://academic.oup.com/cid/article/64/suppl_2/S98/3782679 by guest on 15 April 2021 S102 • CID 2017:64 (Suppl 2) • Wong et al hospitals joined the accreditation to demonstrate achievement of standards through independent external peer assessment. Hospitals need to have well-established infection control systems with effective governance, a clear reporting system, and welltrained infection control staff to be accredited. With the infection control preparedness in place, there has been no secondary nosocomial AI transmission in our healthcare facilities [11].
Laboratory Preparedness
Rapid diagnosis is essential to guiding therapy, taking appropriate isolation precautions, and initiating an effective contact investigation. In 2003, the SARS outbreak reignited the need for rapid molecular diagnostic tests. AI H5N1 and H9N2 also struck the territory in the same year [12, 13]. In the H5N1 family cluster, reverse-transcription PCR (RT-PCR) for influenza was positive in only 1 of the 2 nasopharyngeal aspirates (NPAs) gathered from each patient. Further quantitative RT-PCR study revealed that patients who suffered from H5N1 had lower viral load detectable from NPA than patients who suffered from H3N2 [12].
In view of a strong need for an ongoing system for monitoring emerging viruses and refining the diagnostic tools in a timely manner, the HA Rapid Diagnostic Laboratory Network on Influenza was established in 2004. Equipment, staffing and training, protocol development, reagent stockpile, and quality of results were addressed within the network of 5 HA laboratories. Quality control was of the utmost importance and was part of an External Quality Assurance Program in collaboration with the public health laboratory of the CHP.
From 2007 to 2009, 4 more cases of H9N2 emerged. Diagnosis of AI was then largely dependent on molecular diagnosis. Influenza A H9, which was diagnosed as the specimen, was negative for the pandemic influenza A H1, human H1 and H3 genes by RT-PCR and was subsequently positive for the H9 gene only [14].
A true testimony to the importance of the Laboratory Network arose in April 2009, when the human swine influenza pandemic arrived. Its transmissibility and virulence were virtually unknown at the beginning of the pandemic. The Laboratory Network faced the challenge and provided accurate diagnosis with a short turnaround time. During the 1-year period from May 2009 to April 2010, the Laboratory Network performed around 127 000 influenza A H1 (swine) RT-PCR tests for 103 000 patients. Among them, 16 800 patients were diagnosed with swine influenza. The turnaround time was less than 24 hours for all swine influenza requests. The Laboratory Network constantly shared experience, reagents, and even equipment to ensure a high level of performance. The importance of the Laboratory Network was recognized, and the number of laboratories within the network grew from 5 to 8 after the pandemic.
The Laboratory Network’s latest challenge was the emergence of H7N9. Since the first case of H7N9 was diagnosed in China in March 2013 [15], the network has performed real-time RT-PCR tests for all suspected AI cases and diagnosed 16 cases from 2013 to 2016.
Clinical Management Preparedness
A Taskforce of Clinical Management on Infections, comprising experts in adult and pediatric infectious diseases, clinical microbiology, respiratory medicine, and intensive care, oversees the development and review of guidelines and protocols on management of novel influenzas in the HA. In line with recommendations from international agencies, oseltamivir, a neuraminidase inhibitor, is the first-line antiviral drug for treatment of AI in Hong Kong [16]. Early initiation of oseltamivir may reduce the risks of severe influenza infection, complications, and mortality [17–22].
In addition to treatment of established infection, oseltamivir is recommended as post-exposure presumptive therapy for AI in some situations [23, 24]. A risk stratification approach is adopted, taking into account the risk factor(s) of exposed persons for severe infection and complications as well as circumstances of exposure that have implications on risk of transmission.
In Hong Kong, the Scientific Committee on Emerging and Zoonotic Diseases (SCEZD) under the CHP, with experts from hospitals, academia, public health, and other related sectors, meets regularly and on an ad hoc basis to discuss management strategies for AI and to make recommendations. Many health organizations including the WHO have been advocating for antiviral stockpiling and pandemic preparedness [25]. This strategy has been shown to be cost effective [26, 27]; the SCEZD recommended antiviral stockpiling [28].
Since 2007, we have successfully diagnosed and treated 23 novel influenza cases. Seven patients were admitted to the intensive care unit (ICU), and among them, 5 needed extracorporeal membrane oxygenation.
Development of Surge Capacity
In addition to early detection, diagnosis, isolation, and notification, the HA also has preparedness planning in case of a large number of infectious diseases that require hospital admission. As the occupancy rates in acute medical wards of public hospitals are constantly very high, at greater than 90% [29], any acute increase in admission due to an escalating outbreak may cause strain on the service. To prepare for potential outbreaks, contingency plans have been developed by mobilize beds, facilities, and staffing. At the department and hospital levels, internal staff mobilization plans have been established to prepare for the surge in patients in inpatient, outpatient, and community-based settings, which is tested annually during the peak winter influenza season [30].
To meet service needs, a registry of professional and trained staff with experience on infectious disease wards and in ICUs Downloaded from https://academic.oup.com/cid/article/64/suppl_2/S98/3782679 by guest on 15 April 2021 From SARS to Avian Influenza Preparedness in Hong Kong • CID 2017:64 (Suppl 2) • S103 is maintained. A guideline on human resource policies and arrangements during serious and emergency response levels has been promulgated.
The IDC was established at Princess Margaret Hospital in 2008. It is a standalone building with 108 negative-pressure single rooms equipped with high-efficiency particulate air filters; isolation beds; biosafety level 3 laboratory, radiodiagnostics, and imaging services; a control and command center; and an operation theatre, delivery suite, and ICU. In addition to providing clinical management, the IDC also serves as a training and research center for infectious diseases and infection control professionals. In the contingency plan, the first 20 cases of confirmed AI would be managed in the IDC. Subsequently, 7 major hospitals will take care of the extra 20 cases each, depending on geographical locations. With this arrangement, a single center will not be overwhelmed when there is an escalating epidemic [4]. The amount of ICU equipment, including beds, ventilators, portable X-ray machines, and extracorporeal membrane oxygenation capacity, is measured regularly to plan for mobilization.
To alleviate the strain on already overburdened AEDs, there is a plan in place for converting existing general outpatient clinics in each district to designated clinics where febrile patients are cared for during large-scale outbreaks. Discussions are underway on collaboration with private hospitals and doctors when there is a staffing shortage during a crisis. Workshops have been organized to equip staff with psychological tools that can be used to build up resilience when faced with a major crisis. After SARS, we were bombarded by challenges of AI and other EIDs, which tested our preparedness and surge capacity (Figure 2).
Effective Communication Preparedness Plans
Effective, timely, and accurate communication with staff, patients, and public is essential. Such communication ensures that healthcare workers are aware of the situation and makes it possible to share essential information with other governmental departments and private healthcare sectors.
When an AI case is imported from mainland China, the CHP would inform China’s health authority regarding necessary investigation and follow-up action. The CHP would also notify the WHO and the health authority of Macau.
Since 2007 the CHP has issued 31 letters to doctors and hospitals on AI in order to keep them up to date on the latest situation and to provide them with clear health advice on infection control that corresponds to the response level. Public health announcements would be published via public media and the CHP web site. The CHP has issued 469 government press releases related to AI preparedness since 2007.
CONCLUSION
Here, we provide a brief account of measures taken by the public hospital system in Hong Kong related to its AI preparedness. AI poses a continuous threat to humans, echoing the recommendations of WHO and other organizations on influenza at the human–animal interface. The Preparedness Plan for Influenza Pandemic can be used as a model to develop preparedness and response plans for other EIDs.
Notes
Author contributions. A. T. Y. W., H. C., S.-h. L., E. K. H., K. S. L., C. K. C. L., R. F. Y. C., O. T. Y. T., K. W. C., Y. W. K., A. Y. H. T., and D. N. C. T. are members of the writing group who formulated the idea and wrote the article. V. C. C. C. is a member from Central Committee on Infectious Diseases and Emergency Response and gave final comments on the article. All authors read and approved the manuscript.
Acknowledgments. The authors thank the chief executive and members of the director’s meeting of the HA for supporting the idea of sharing the experience of AI preparedness in Hong Kong. The authors thank members of the Central Committee on Infectious Diseases and Emergency Response and members of the Task Force of Infection Control of the HA for providing comments on the manuscript. We further acknowledge the Surveillance and Epidemiology Branch of CHP for providing surveillance data on AI. Finally, we thank Luisa Chan, Peggy Tam, Kelvin Yu, and Eliza Chan for logistic support.
Disclaimer. The funding source did not play a role in writing of this manuscript or in the decision to submit this manuscript for publication.
Supplement sponsorship. This article appears as part of the supplement “Infection Prevention in Asia Pacific,” sponsored by the Infectious Diseases Association of Thailand (IDAT) with additional author sponsorship.
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- Hung LS. The SARS epidemic in Hong Kong: what lessons have we learned? J R Soc Med 2003; 96:374–8.
- SARS Expert Committee, the Government of the Hong Kong Special Administrative Region. SARS in Hong Kong: from experience to action : a summary report of the SARS Expert Committee. 2003. Available at: http://www.sars-expertcom.gov.hk/ english/reports/summary/reports_sumrpt.html. Accessed 9 September 2016.
- The Government of the Hong Kong Special Administrative Region. Preparedness Plan for Influenza Pandemic. 2014. Available at: http://www.chp.gov.hk/files/pdf/ erib_preparedness_plan_for_influenza_pandemic_2014_eng.pdf. Accessed 11 September 2016.
- Working group for reviewing contingency plans for AI & SARS. Review on HA’s Preparedness for Influenza Pandemic. 2012. Available at: http://www.ha.org.hk/ haho/ho/cad_bnc/HAB-P175.pdf. Accessed 11 September 2016.
- Leung Y-H, To M-K, Lam T-S, Yau S-W, Leung O-S, Chuang S-K. Epidemiology of human influenza A(H7N9) infection in Hong Kong. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. doi:10.1016/j.jmii.2015.06.004.
- Liu B, Havers F, Chen E, et al. Risk factors for influenza A(H7N9) disease—China, 2013. Clin Infect Dis 2014; 59:787–94.
- Cheung NT, Fung KW, Wong KC, et al. Medical informatics—the state of the art in the hospital authority. Int J Med Inform 2001; 62:113–9.
- Cheng VC, Chan JF, To KK, Yuen KY. Clinical management and infection control of SARS: lessons learned. Antiviral Res 2013; 100:407–19.
- Centers for Disease Control and Prevention. Interim guidance for infection control within healthcare settings when caring for confirmed cases, probable cases, and cases under investigation for infection with novel influenza A viruses associated with severe disease. 2016. Available at: http://www.cdc.gov/flu/avianflu/ novel-flu-infection-control.htm. Accessed 21 September 2016.
- World Health Organization. Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. Geneva: WHO, 2014. Available at: http://apps.who.int/iris/handle/10665/112656. Accessed 21 September 2016.
- Cheng VC, Tai JW, Lee WM, et al. Infection control preparedness for human infection with influenza A H7N9 in Hong Kong. Infect Control Hosp Epidemiol 2015; 36:87–92.
- Peiris JS, Yu WC, Leung CW, et al. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 2004; 363:617–9. Downloaded from https://academic.oup.com/cid/article/64/suppl_2/S98/3782679 by guest on 15 April 2021 S104 • CID 2017:64 (Suppl 2) • Wong et al
- Butt KM, Smith GJD, Chen H, et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol 2005; 43:5760–7.
- Cheng VC, Chan JF, Wen X, et al. Infection of immunocompromised patients by avian H9N2 influenza A virus. J Infect 2011; 62:394–9.
- World Health Organization. Avian influenza. 2014. Available at: http://www.who. int/mediacentre/factsheets/avian_influenza/en/. Accessed 9 September 2016.
- Centers for Disease Control and Prevention. Interim guidance on the use of antiviral medications for treatment of human infections with novel influenza A viruses associated with severe human disease. 2016. Available at: http://www.cdc.gov/flu/ avianflu/novel-av-treatment-guidance.htm. Accessed 2 September 2016.
- Adisasmito W, Chan PK, Lee N, et al. Effectiveness of antiviral treatment in human influenza A(H5N1) infections: analysis of a global patient registry. J Infect Dis 2010; 202:1154–60.
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A. (H5N1) Virus. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–73.
- Liem NT, Tung CV, Hien ND, et al. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004–2006. Clin Infect Dis 2009; 48:1639–46.
- Uyeki TM. Human infection with highly pathogenic avian influenza A (H5N1) virus: review of clinical issues. Clin Infect Dis 2009; 49:279–90.
- Chan PK, Lee N, Zaman M, et al. Determinants of antiviral effectiveness in influenza virus A subtype H5N1. J Infect Dis 2012; 206:1359–66.
- Kandun IN, Tresnaningsih E, Purba WH, et al. Factors associated with case fatality of human H5N1 virus infections in Indonesia: a case series. Lancet 2008; 372:744–9.
- Centers for Disease Control and Prevention. Interim guidance on follow-up of close contacts of persons infected with novel influenza A viruses associated with severe human disease and on the use of antiviral medications for chemoprophylaxis. 2016. Available at: http://www.cdc.gov/flu/avianflu/novel- av-chemoprophylaxis-guidance.htm. Accessed 2 September 2016.
- World Health Organization. Avian influenza A (H7N9) virus: Post-exposure antiviral chemoprophylaxis of close contacts of a patient with confirmed H7N9 virus infection and/or high risk poultry/environmental exposures. 2014. Available at: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/13_ January_2013_PEP_recs.pdf. Accessed 13 September 2016.
- World Health Organization. Pandemic influenza preparedness and response: a WHO guidance document. Geneva: WHO; 2009.
- Siddiqui MR, Edmunds WJ. Cost-effectiveness of antiviral stockpiling and near-patient testing for potential influenza pandemic. Emerg Infect Dis 2008; 14:267–74.
- Lugnér AK, Postma MJ. Investment decisions in influenza pandemic contingency planning: cost-effectiveness of stockpiling antiviral drugs. Eur J Public Health 2009; 19:516–20.
- Scientific Committee on Emerging and Zoonotic Diseases, Centre for Health Protection, the Government of the Hong Kong Special Administrative Region. Summary of recommendations on antiviral stockpiling for influenza pandemics. 2014. Available at: http://www.chp.gov.hk/files/pdf/summary_of_recommendations_ on_antiviral_stockpiling_for_influenza_pandemics.pdf.
- Government of the Hong Kong Special Administrative Region. Legistlative Council question 3: nursing manpower in public hospitals. Available at: http:// www.info.gov.hk/gia/general/201605/18/P201605180556.htm. Accessed 29 September 2016.
- Government of the Hong Kong Special Administrative Region. Key statistics on service demand of A&E Departments and occupancy rates of medical wards in public hospitals. 2016. Available at: http://www.info.gov.hk/gia/general/ 201603/22/P201603220310.htm. Accessed 15 September 2016.
